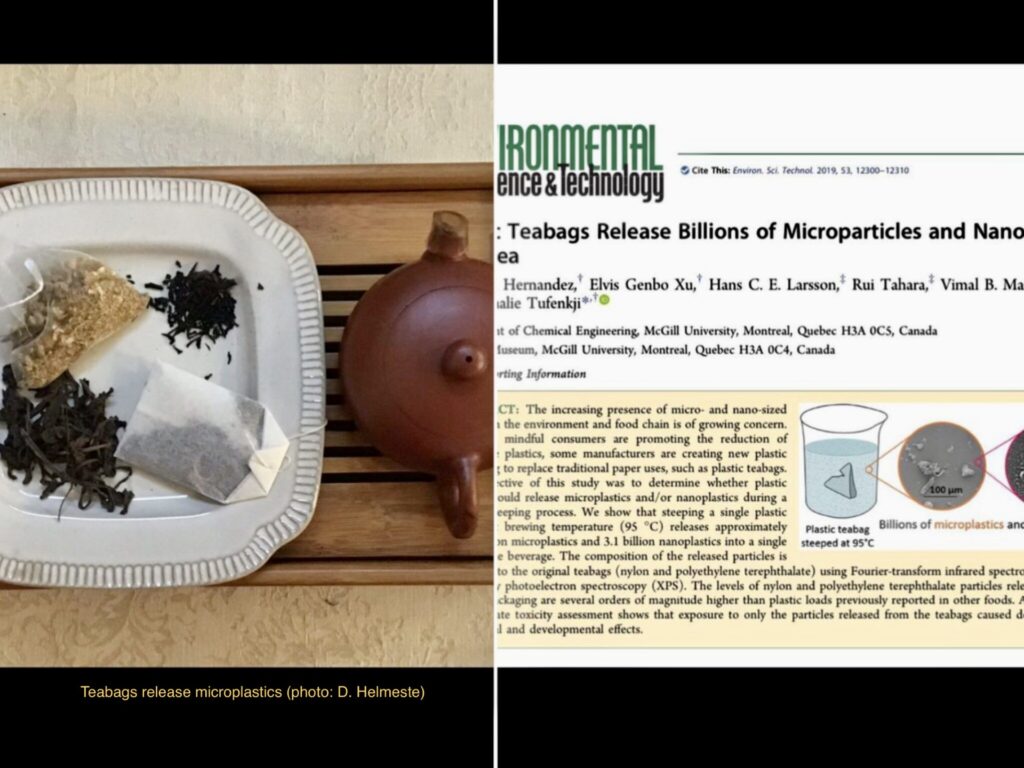
Where can we get a good sample of microplastics for study? Apparently, you need look no further than your teacup, if you use tea bags. According to L. Hernandez and colleagues at McGill University in Montréal (Environ. Sci. Technol. 53: 12300, 2019), modern tea bags that use plastics can release billions of microplastics into a single cup of beverage when the tea is steeped at 95 degrees Celsius. Despite this problem, one can still find plastic tea bags available for sale, with no warning signs on the packaging. Luckily, switching to loose leaf tea or a cloth-based tea bag avoids this problem.
Removing microplastics
If microplastics are already in the water supply, boiling "hard" water (greater than 120 mg/L CaCO3 [calcium carbonate]) can remove at least 80% of polystyrene, polyethylene, and polypropylene microplastics, between the sizes of 0.1 and 150 micrometres. Zimin Yu (Guangzhou Medical University) and colleagues found that the elevated temperatures promote calcium carbonate nucleation on microplastics, resulting in aggregation of the microplastics within calcium carbonate incrustants [crust residue] (Environ. Sci. Technology Lett. 11,3: 273-279, 2024).
Filtering water is another option, but may be more expensive, especially when using filters with 0.2 to 1 micrometre pore sizes. Understanding these advances in the field should help devise new clean water strategies.

Some notable inventions
Looking at recent science fair results for younger students, two handy inventions deserve our attention. D. Goldshleger, a grade six student in California, invented a pocket-sized vacuum cleaner to suck up small particles from his desk. It has a very simple, easy-to-put-together design, using a plastic syringe, a direct current motor, a battery pack, and a fan (cut from an aluminum can) attached to the motor. The vacuum was most efficient when the fan blades had an angle of 25 degrees. The attractive features of this project are its simplicity and inexpensiveness. It also shows how understanding the scientific principles behind these projects can lead to new, easy-to-use products.

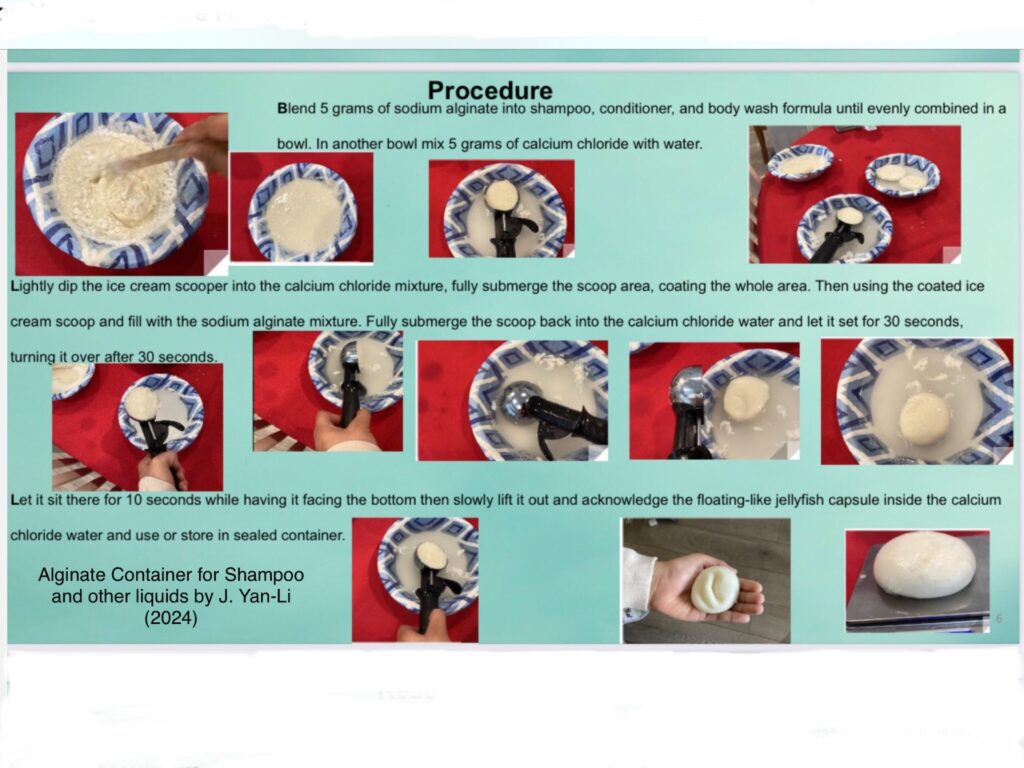
J. Yan-Li, also a grade six student in California, studied the idea of using alginate as a replacement for plastic containers to hold shampoo and other liquids. To make the alginate container, the student combined sodium alginate and calcium chloride to make a soft casing that lasts for several weeks and is biodegradable. It's a simple procedure and produces quick results.
Hopefully these clever ideas can inspire new projects among our global Estonian community. Now that summer is only three months away, now is a good time for students to start looking for summer opportunities to visit or work at places that could lead to new startups or business ventures. Here's wishing you happy adventures ahead!
Become a subscriber to continue reading!
Every week we bring you news from the community and exclusive columns. We're relying on your support to keep going and invite you to subscribe.
Starting from $2.30 per week.